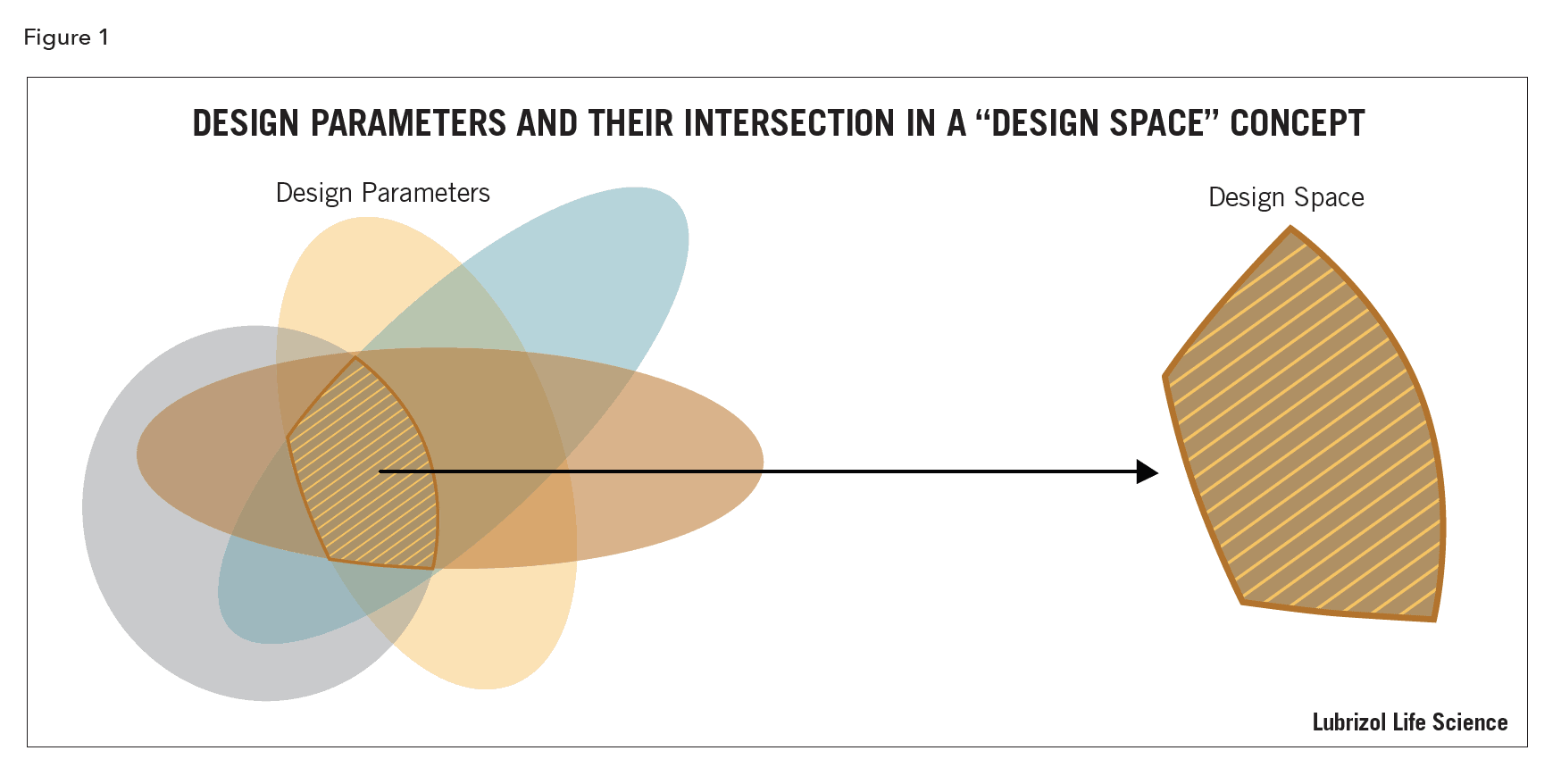
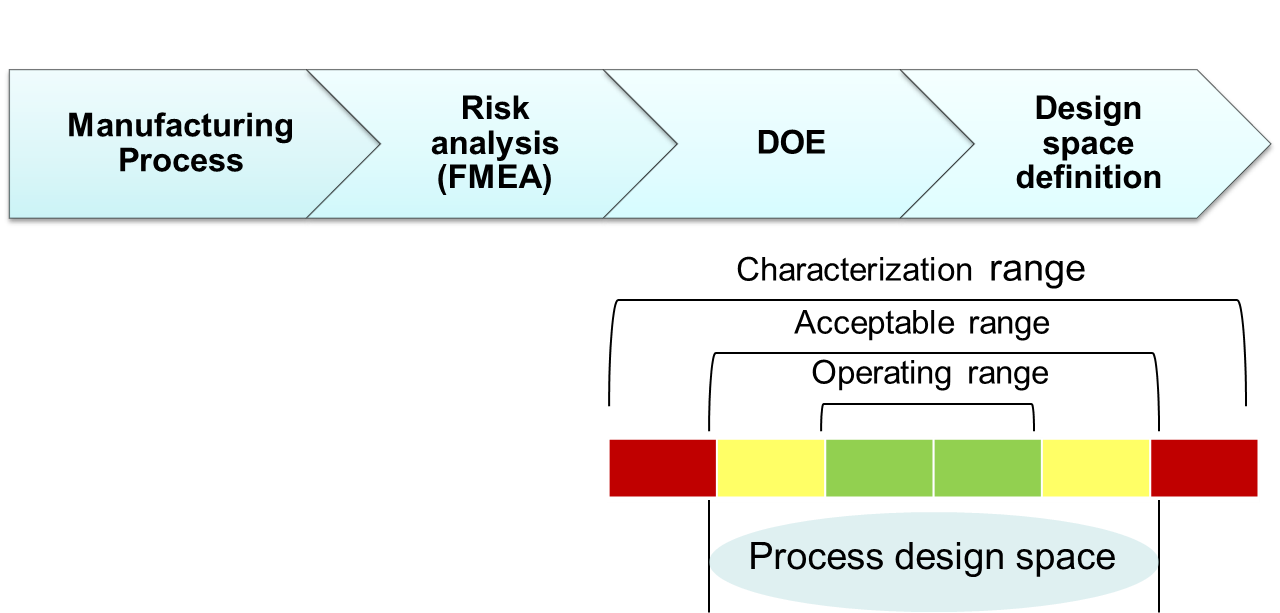
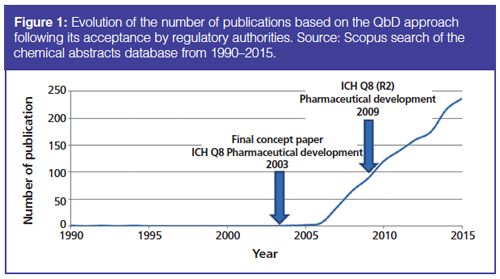
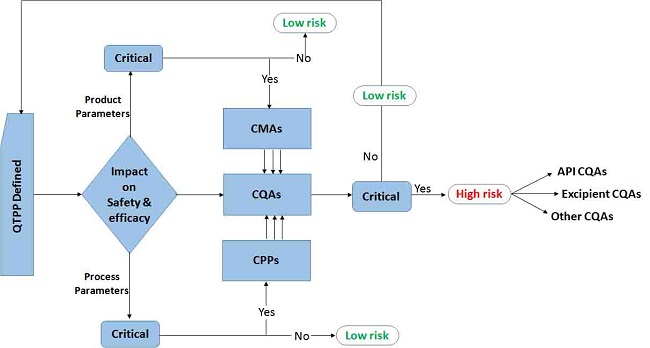
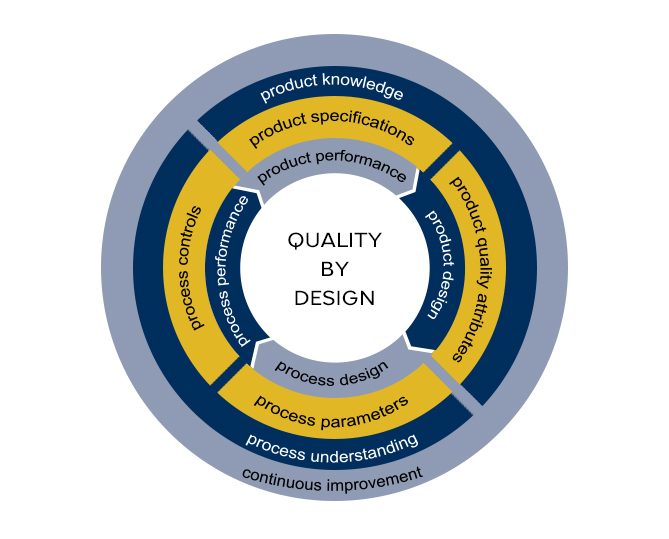
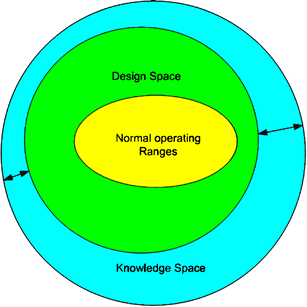
QbD Framework – in 2 Minutes (for Scientists) – Quality by Design for Biotech, Pharmaceutical and Medical Devices
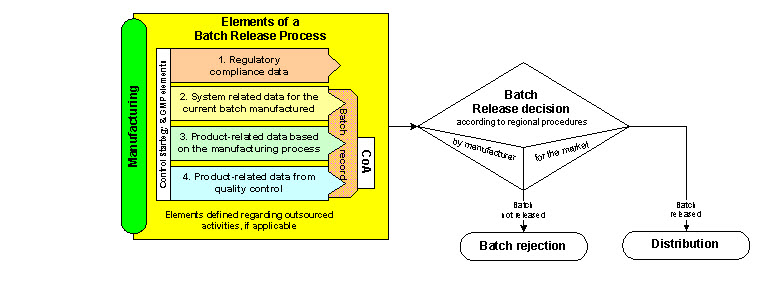
Q8, Q9, & Q10 Questions and Answers -- Appendix: Q&As from Training Sessions (Q8, Q9, & Q10 Points to Consider) | FDA
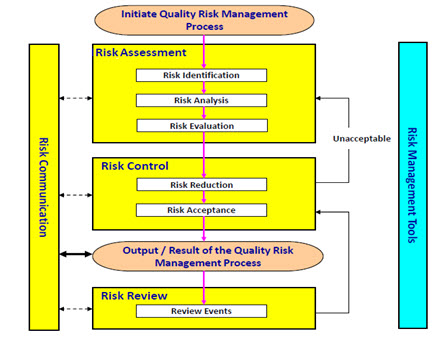
QUALITY BY DESIGN (QbD) IN PHARMACEUTICAL INDUSTRY: TOOLS, PERSPECTIVES AND CHALLENGES | PharmaTutor

International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Implementation of ICH Q8, Q9, Q ppt download
![PDF] A quality by design approach on pharmaceutical development of orally disintegrating tablet of Diazepam | Semantic Scholar PDF] A quality by design approach on pharmaceutical development of orally disintegrating tablet of Diazepam | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/beb45c06f39cac3f1af99a8f5f3993daeb756541/24-Figure5-1.png)
PDF] A quality by design approach on pharmaceutical development of orally disintegrating tablet of Diazepam | Semantic Scholar
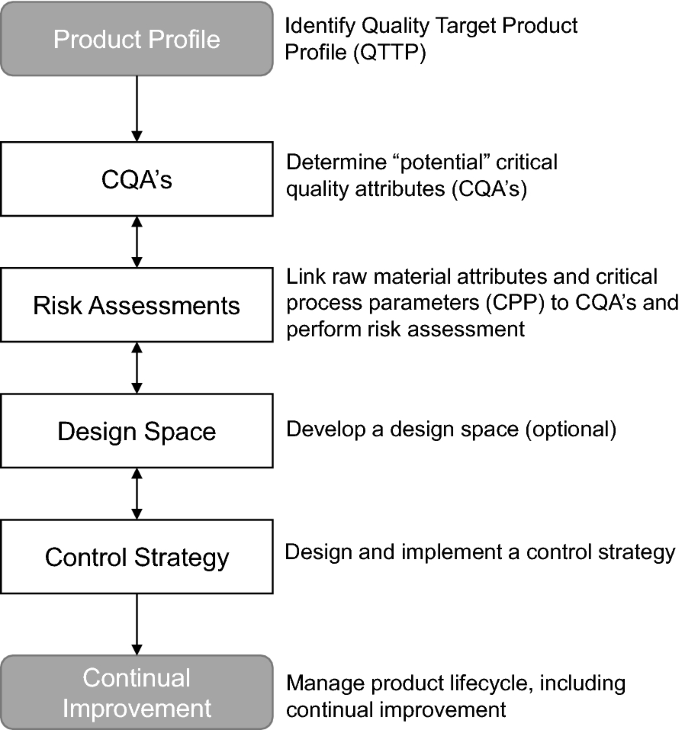
Implementation of Quality by Design (QbD) Principles in Regulatory Dossiers of Medicinal Products in the European Union (EU) Between 2014 and 2019 | SpringerLink